
During the pandemic, isopropyl rubbing alcohol became one of the most in-demand household products in the world. As soon as stores ran out of Lysol, disinfectant sprays, and hand sanitizer, the next best thing was medical-grade rubbing alcohol.
Isopropyl rubbing alcohol’s widespread use and popularity led many of its users to look into why the liquid is such a good cleaning and disinfectant agent.
So is isopropyl alcohol polar? Isopropyl rubbing alcohol is nonpolar. Although there is a small region of the molecule that remains polar, the majority of the Isopropyl alcohol molecule is non-polar which allows it to dissolve oils.
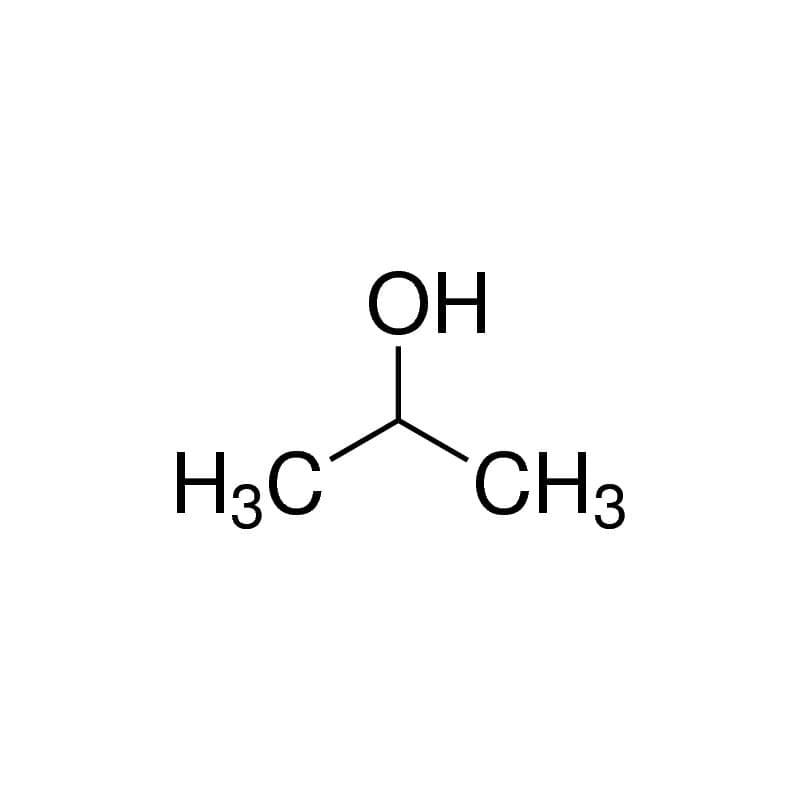
Confusing terms huh, don’t worry. I’m not going to ask you to go back and re-read your high school textbooks.
Instead, I’m going to break down why the polarity of isopropyl alcohol matters and how it allows the substance to find its use for everything from cleaning messes to killing bacteria.
Welcome to alcohol science 101!
The Polarity Of Isopropyl Alcohol and Why It Matters
Almost everybody has a bottle of isopropyl rubbing alcohol sitting around their home, office, or garage.
As far as cleaning agents go, it’s incredibly cheap (only cost a few dollars for a bottle) and effective.
It’s also a very versatile compound useful for a variety of different things, including:
- Disinfecting surfaces.
- Killing mold and mildew.
- Cleaning windows and glass.
- Cleaning up oily messes and spills.
- Removing old car wax and preparing paint.
- … and more!
While nobody will deny how effective and versatile rubbing alcohol is, almost nobody actually understands how or why rubbing alcohol is able to do what it does. As I mentioned before, it all comes down to molecular polarity.
That being said, let’s use that as a good place to start our discussion on isopropyl rubbing alcohol’s polarity and why it matters.
What Is Molecular Polarity?
The easiest way to describe molecular polarity is to describe a traditional magnet. In a magnet, one side holds a positive charge while the other holds a negative charge.
This is why magnets attract each other and why metals stick to magnets. In much the same way, the atomic structure of a molecule determines whether it is polar or not:
- In polar molecules, one or more atoms “hog” the electrons, resulting in one side of the molecule having a positive charge, while the other has a negative charge.
- non-polar molecules, atoms share electrons evenly, resulting in a negative charge.
In other words, in polar molecules, the positively charged electrons jump onto one element, leaving the other elements with a deficit of electrons (or negatively charged).
Whereas in non-polar molecules, the electrons may trade places with each other and move from one element to another, but they will never leave one side of the compound lacking electrons.
“Like Dissolves Like” and Miscibility
By now, you’re probably thinking, “well that’s cool and all, but how does this apply to isopropyl alcohol’s general usefulness?”
That brings us to our next point- miscibility. This term describes the ability of two liquids to mix together and form a homogenous substance. Or, in other words, two liquids’ ability to dissolve into each other.
Take oil, water, and alcohol, for example. Oil is non-polar, water is polar, and alcohol is mostly non-polar. If you try to mix oil and water together, you know what happens; the oil floats in weird little bubbles across the surface of the water. In other words, the two substances are not miscible.
Now, try mixing alcohol with oil; you’ll find that the oil’s thickness quickly breaks down and that it can be easily wiped away. This means that oil and alcohol are miscible.
If you remember your high school chemistry class, then you’ll remember that “likes dissolve likes” This means that to dissolve a non-polar substance like oil, you’ll need another non-polar substance like alcohol. This is why you can’t clean up oil residue with plain water (it will only make it even messier).
Is All Alcohol Non-Polar?
I came across this question while browsing through Reddit the other day. The answer is, yes all alcohol has the same basic chemical structure and molecular properties.
The main difference is the method that is used to make the alcohol. That being said, ethanol or even denatured alcohol are just as good at dissolving non-polar substances as isopropyl alcohol is.
What Happens When You Mix Water and Isopropyl Alcohol?
Now, this is where many people have confusion about isopropyl rubbing alcohol and start asking questions. Since alcohol is largely non-polar (which allows it to mix with and dissolve an oil-based substance), it shouldn’t be able to mix with water, right?
Yes, isopropyl rubbing alcohol is mostly non-polar. However, that “mostly” is what makes all of the difference. Unlike a purely non-polar substance, alcohol molecules are partly polar. This is what allows them to be mixed down with water.
For example, when you go to the store to purchase rubbing alcohol, you’ll probably notice that they all have different strengths (e.g., 50% alcohol, 70% alcohol, 95% alcohol). These bottles are not pure rubbing alcohol; they’re mixed with water.
For instance, the 70% rubbing alcohol consists of a mixture of 70% isopropyl alcohol and 30% water. The ratio of alcohol to water determines the strength of the mixture.
The higher the percentage of alcohol, the more non-polar the compound is, and the better it can clean away oils.
How Does Isopropyl Alcohol Kill Germs and Bacteria?
Alcohol’s combination of non-polar and polar molecules is what allows it to kill germs and bacteria so easily.
Unlike many other germicides on the market, isopropyl alcohol is non-toxic and is able to quickly eliminate deadly bacteria in seconds. This is because bacteria consist of both polar and non-polar molecules as well.
Once the alcohol comes into contact with the bacteria, the non-polar and the polar molecules both dissolve each other, effectively “killing” the bacteria.

My name is Logan, and I’m a 36-year-old dad who owns a small pressure-washing company in the suburbs of Atlanta, Georgia. My main goal with rubbing-alcohol.com is to show you how versatile isopropyl rubbing alcohol can be! I hope. You find it useful.
